Ratko Djukanovic, Stephen Holgate & Donna Davies: Three COVID drug professors become overnight millionaires as principals of Synairgen’s experimental drug, SNG001 positive trial results this week. But what obstacles & challenges lie ahead?
Are three British university professors on the cusp of saving mankind, while also making themselves very rich in the process?
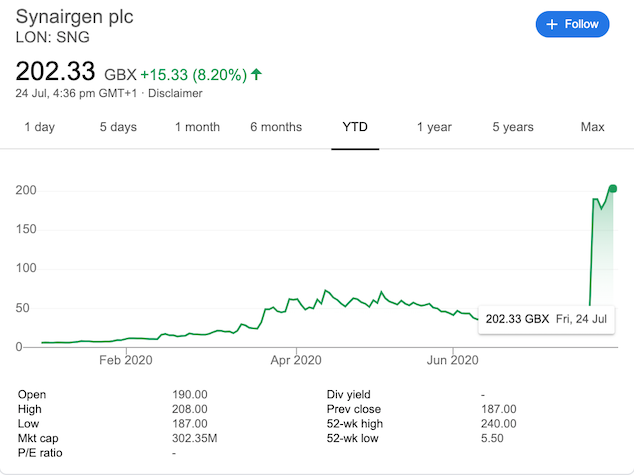
Ratko Djukanovic, 65, Stephen Holgate, 73, and Donna Davies, 67, have become overnight millionaires after a ‘major breakthrough’ in their coronavirus drug saw shares of their Southampton–UK based company Synairgen jump 3,000 per cent almost overnight following the release of ‘successful results’ of a drug to treat COVID-19 earlier this week reported The Guardian.
At the start of this year, shares in Synairgen were valued at less than 6p. By July 17, the biotech firm’s share price had risen to 36p, and by close of play on Friday the shares had shot up to £2.04 ($2.59 USD). Speculators believe that share prices could double in coming months.
Professor in medicine Djukanovic, saw his 0.56 per cent stake in the company jump in value in one day from around £300,000 ($377K USD) to £1.6m ($2m USD) The 0.59 per cent stake held by Holgate, 73, a professor of immunopharmacology, rose to £1.7m ($2.2m USD).
It is thought the third founder and professor of respiratory cell and molecular biology, Donna Davies, holds a similar-sized stake through another company.
Potential COVID-19 treatment?
In a study of 101 people, coronavirus patients who were given a special formula of the professor’s interferon drug, SNG001, were two or three more times more likely to recover than those given a placebo.
Patients given the drug directly into their airways via a nebuliser, a powerful inhaler, were 79 percent less likely to become seriously ill with the disease.
Told Richard Marsden, Synairgen’s chief executive following the release of results this week: ‘For those on the drug breathlessness was ‘markedly reduced’.
‘We are all delighted with the trial results announced today, which showed that SNG001 greatly reduced the number of hospitalised COVID-19 patients who progressed from ‘requiring oxygen’ to ‘requiring ventilation’. It also showed that patients who received SNG001 were at least twice as likely to recover to the point where their everyday activities were not compromised through having been infected by SARS-CoV-2. In addition, SNG001 has significantly reduced breathlessness, one of the main symptoms of severe COVID-19. This assessment of SNG001 in COVID-19 patients could signal a major breakthrough in the treatment of hospitalized COVID-19 patients. Our efforts are now focused on working with the regulators and other key groups to progress this potential COVID-19 treatment as rapidly as possible.’
Adding, ‘[For them -referring to the three professors involved in the trial] it doesn’t get better than seeing a drug you created treating real patients, and the side-effect of that is you make money… If people are clever and find something useful, they should get rewarded economically.’
While no deaths were reported in the SNG001 group, three subjects in the placebo arm died.
The company added that the likelihood of hospital discharge increased with its treatment candidate in patients who had more severe disease at the time of admission, but the difference did not achieve statistical significance.
Three university professors become overnight MILLIONAIRES after finding ‘major breakthrough’ in Covid drughttps://t.co/1VIABQv0M6
— Daily Mail U.K. (@DailyMailUK) July 25, 2020
Substituting for missing vital protein
The three professors first came into prominence in 2004 after they discovered people with asthma and chronic lung disease lack a protein called interferon beta. The protein can help the body fight off the common cold. If the missing protein is replaced the body’s natural defenses are better able to beat a viral infection.
The clinical trial results were published on July 21, and by lunchtime Synairgen shares were up by 540 per cent.
The company directors’ combined 2.6 per cent stake has now been valued at more than £7million ($9m USD).
Marsden, who holds 0.3 per cent of stocks, said the 204p share price at close of day Friday was ‘reasonable’.
‘It is a major breakthrough in the treatment of hospitalised Covid-19 patients,’ Marsden who holds 0.3 percent of shares told the Guardian. ‘We couldn’t have expected much better [trial] results than these.’
The trial has now been expanded to patients suffering with milder coronavirus symptoms at home, in an effort to lower the number of cases in hospitals.

What lies ahead for Synairgen?
Drug manufacturer Rentschler has now been ordered to start producing more than a million doses of the drug ahead of an anticipated second wave in the winter.
Synairgen started setting up their clinical trial in February and March in a bid to ensure a drug was ready by the time coronavirus infiltrated major states starting in April.
The next stage for Synairgen is gaining the approval of worldwide medical regulators so the treatment can be brought to market.
Independent experts previously said Synairgen’s trial would ‘represent by far the biggest breakthrough in Covid-19 treatment to date’ if the results are verified.
The only drug scientifically proven to treat the disease at present is a £5 steroid known as dexamethasone, which slashes death rates by up to a third.
Southampton-based Synairgen is a publicly traded firm, meaning it was obligated to release the preliminary results due to stock market rules.
The trial involved 101 Covid-19 patients who had been admitted at nine UK hospitals and required oxygen support. Half of the recruits were given the drug, while the rest took a placebo.
The trial was carried out on a double blind basis, meaning neither the researchers nor the 101 patients knew who was receiving SNG001.
Three people died after being randomly assigned the placebo, while there were no deaths among those who received the drug, Synairgen said.
Cautious optimism: Synairgen’s positive results from trial of interferon beta in COVID-19 −Patients who received SNG001 had a 79% lower risk of severe disease & more than twice as likely to recover from COVID-19- compared to placebo controls
Safety data still being processed— Paul Hertzog (@pjhpauljh) July 22, 2020
101 COVID-19 patients given new interferon beta drug, SNG001 (press release) via a nebuliser, were 79% less likely to develop severe disease and had reduced breathlessness. No formal peer reviewed paper yet – but expert reaction cautiously positive. https://t.co/4hslwd6ube
— Ritu Dhand (@RituDhand) July 25, 2020
Cautious optimism
Studies have shown key high risk groups for Covid-19, including older people and those with some chronic diseases have lower levels of interferon beta.
Separate trials in asthmatic patients have shown SNG001 is well-tolerated, boosts the lungs anti-viral defences and helps lung function during cold or flu infection.
The inhaler turns SNG001 into a fine mist so it can be inhaled deep into the lungs, with the hope it will trigger a stronger, more targeted anti-viral response.
Interferon beta is already used as an injection to boost the immune response of people with multiple sclerosis.
The trial’s chief investigator, Tom Wilkinson, professor of respiratory medicine at the University of Southampton, said if the results are replicated in larger studies it will be ‘a game changer’.
He added: ‘The results confirm our belief that interferon beta, a widely known drug that, by injection, has been approved for use in a number of other indications, has huge potential as an inhaled drug to be able to restore the lungs’ immune response, enhancing protection, accelerating recovery and countering the impact of Sars-CoV-2 virus.’
Stephen Holgate CBE, professor of immunopharmacology at the University of Southampton, said recognizing that the coronavirus ‘is known to have evolved to evade the initial anti-viral response of the lung’ was a valuable insight.
‘Our treatment of giving high local concentrations of interferon beta, a naturally occurring antiviral protein, restores the lungs ability to neutralise the virus,’ added Professor Holgate, who is also the co-founder of Synairgen.
He said it would work on ‘any mutation of the virus or co-infection with another respiratory virus such as RSV or influenza, as could be encountered in the winter if there is a resurgence of Covid-19’.
Reservations exist
Reacting to the findings, Professor Francois Balloux, a geneticist at University College London, tweeted: ‘Preliminary results from a clinical trial suggest interferon beta reduces the risk of developing severe #COVID19 disease by 79 per cent.
‘If confirmed, this would represent by far the biggest breakthrough in #COVID19 treatment to date.’
Naveed Sattar, professor of metabolic medicine at the University of Glasgow, added: ‘The results seem very impressive, and although accepted that the trial is small with just over 100 participants, a 79 per cent reduction in disease severity could be a game changer.
‘It would be good to see the full results once presented and peer-reviewed to make sure they are robust and the trial conduct was rigorous.
‘Also, with small numbers comes less certainty on the true level of benefit, or whether benefits vary between people with differing risk characteristics. Such work would require a larger trial but, even so, these results are very exciting.’
But reservations do exist, with Professor Steve Goodacre, an expert in emergency medicine at the University of Sheffield, saying: ‘These results are not interpretable.
‘We need the full details and, perhaps more importantly, the trial protocol. The trial should have been registered and a protocol made available before any analysis was undertaken.’
Synairgen will now have to present its findings to medical regulators around the world before it is approved.
Health chiefs will review the findings and decide whether to approve the treatment so doctors can treat Covid-19 patients with it.
The firm’s chief executive Richard Marsden told the BBC it would be able to deliver a ‘few hundred thousands’ of doses each month by the winter.
Because the study was quite small — only involving 100 patients — the trial may have to be scaled up before getting approval.
This process could take months, although governments around the world might be open to fast-tracking the drug if they are impressed by the findings.
British ministers have approved the Ebola drug remdesivir for emergency use on patients suffering life-threatening symptoms of Covid — despite evidence about its effectiveness still being mixed.
Only one drug, the £5 steroid dexamethasone, has so far been conclusively proven to treat coronavirus.
The Recovery trial found it reduced the risk of death by 35 per cent for patients on ventilators — the most dangerously ill — and by a fifth for all patients needing oxygen at any point.






